Using OpenAI's GPT API models for Title and Abstract Screening in Systematic Reviews
Mikkel H. Vembye
2025-09-18
Source:vignettes/Using-GPT-API-Models-For-Screening.Rmd
Using-GPT-API-Models-For-Screening.RmdImportant note
Always remember that title and abstract screeening with GPT API
models can be case sensitive. Therefore, see Vembye,
Christensen, Mølgaard, & Schytt (2025) for an overview of
how and when GPT API models can be used for title and abstract (TAB)
screening (find the preprint of Vembye et al. 2025 here). Our most recent
results suggest that the gpt-4o-mini is an effective model
for screening titles and abstracts with performances in many cases on
par with gpt-4. This is a very cheap model (200 times
cheaper than gpt-4). Therefore, to reduce costs, we
recommendation always testing the performance of
gpt-4o-mini before considering other models. For an
overview of additional research on the use of GPT API models for title
and abstract screening, see Syriani et al. (2023, 2024), Guo et al., (2024), and Gargari
et al. (2024). On a related line of research,
Alshami et al. (2023), Khraisha et al. (2024), and
Issaiy et al. (2024) explored the use of the ChatGPT
web browser interface for TAB screening. Based on our experience, we
believe these two lines of research should not be conflated, as they
rely on different GPT models and setups.
Conducting a systematic review is most often resource-demanding. A critical first step to ensure the quality of systematic reviews and meta-analyses herein involves detecting all eligible references related to the literature under review (Polanin, Pigott, Espelage, & Grotpeter, 2019). This entails searching all pertinent literature databases relevant to the given review, most often resulting in thousands of titles and abstracts to be screened for relevance. Manual screening hereof can be a time-consuming and tedious task. However, overlooking relevant studies at this stage can be consequential, leading to substantially biased results if the missed studies differ systematically from the detected studies. To mitigate this resource issue, we demonstrate in this vignette how to use OpenAI’s GPT (Generative Pre-trained Transformer) API (Application Programming Interface) models for title and abstract screening in R. Specifically, we show how to create a test dataset and assess whether GPT is viable as the second screener in your review (cf. Vembye et al., 2025).
At this point, you might ask, why use GPT API models via R instead of simply using ChatGPT for screening? The answer is that using R offer certain advantages:
- Reviewers can easily work with a large number of references,
avoiding the need for manual copy-paste procedures (cf. Khraisha et al.,
2024).
- The screening time can be substantially reduced compared to using the ChatGPT interface, as screening can be performed in parallel. In theory, you can screen 30,000 titles and abstracts per minute.
- It eases model comparison.
- Consistency between the GPT answer for the same title and abstract can be easily tested. You can also conduct multiple screenings and make inclusion judgments based on how often a study is included across a set number of screenings.
- When using the
gpt-4o-mini, it is often cheaper than subscribing to ChatGPT plus. - Research suggest that GPT API models and the ChatGPT yield notably different screening performance, with the latter proving insufficient as an independent second screener.
That said, we would also like to emphasize that screening with the GPT API models should be conducted carefully and always be assisted by humans (human-in-the-loop). Consequently, we do not recommend to use the a GPT API model as a single screener, unless this an absolutely final solution. When using a GPT API model as the second screener, we also recommend completing the human screening before uploading RIS file(s) to R. This allows for immediate comparison once the GPT screening is complete and ensures humans are then not impacted/biased by the GPT decision.
Get Started
For many reviewers, especially those unfamiliar with coding in R, conducting a title and abstract screening in R can seem overwhelming. However, it’s important to emphasize that running your first screening only requires a few relatively simple steps:
- Load and convert your RIS file data to a data frame.
- Handle your API key (this is only necessary the first time you screen).
- Make one or multiple prompts.
- Run test screening(s).
And if the human screening has been conducted (as in the example below):
- Analyze the test screening results via benchmarking (cf. Figure 5).
- Investigate and resolve disagreements.
- Conduct the full title and abstract screening, and repeat step 6 at full scale.
We will walk through each of these steps one by one in the following sections.
Load and Convert RIS File Data to a Data Frame
At this stage, we expect that you have a collection of RIS files
containing titles and abstracts for the references you wish to screen.
You can obtain RIS files in several ways: directly from the research
databases, a Google Scholar search; or by exported them from your
reference management tool, such as EndNote, Mendeley, and RefMan.
Alternatively, you can export RIS files from systematic software tools
such as EPPI-reviewer,
Covidence, MetaReviewer, revtools
(Westgate,
2019), among others. In the example below, we load RIS files
extracted from EPPI-reviewer.
A minor advantage of exporting RIS files from systematic software tools
is that they often add a unique study ID to each reference, making it
easier to track the screening process. However, such IDs are
automatically generated in tabscreen_gpt() if they are
missing.
Load relevant R packages
To get started, we first load all R package needed to construct and run the screening.
# Loading packages
library(AIscreenR) # Used to screen and calculate gpt vs. human performance
library(synthesisr) # Used to load RIS files
library(tibble) # Used to work with tibbles
library(dplyr) # Used to manipulate data
library(purrr) # For loops
library(usethis) # Used to add the API key the R environment (only relevant the first time you screen)
library(future) # Used to conduct screenings in parallelConvert RIS files to data frames
Now, we are ready to conduct the first step of the screening: loading
the RIS file data into R and converting it to a data frame. This can be
done by using read_refs() from synthesisr.
This is an updated version of the read_bibliography()
function from the revtools package that works reliable. In
the below example, we use RIS file data from a review regarding the
effects of the FRIENDS preventive programme on anxiety symptoms in
children and adolescents (Filges, Smedslund, Eriksen, Birkefoss, &
Kildemoes, 2024). Be aware, that this data is used for
illustrative purposes only. In the example, we assume that the human
reviewers have already started screening and identified several relevant
and irrelevant studies, which they will use to create the test data.
Thus, we can load the RIS files, which we downloaded from the
EPPI-reviewer, separately for the in- and excluded RIS files. This
allows us to track the human decision by adding a
human_code variable, where 1 and
0 indicate whether a study is included or excluded,
respectively.
# NOTE: Find the RIS files behind this vignette at https://osf.io/kfbvu/
# Loading EXCLUDED studies
ris_dat_excl <- read_refs("friends_excl.ris") |> # Add the path to your RIS file here
as_tibble() |>
select(author, eppi_id, title, abstract) |> # Using only relevant variables
mutate(
human_code = 0, #Tracking the human decision
across(c(author, title, abstract), ~ na_if(., "NA"))
)
ris_dat_excl
#> # A tibble: 2,765 × 5
#> author eppi_id title abstract human_code
#> <chr> <chr> <chr> <chr> <dbl>
#> 1 Lovat C 911525… "'Ba… "Postna… 0
#> 2 Kohler Maxie 911023… "\"F… "The ar… 0
#> 3 Stevens Eleanor and Wood Jane 911020… "\"I… "'Non-o… 0
#> 4 Essau C and Conradt J and Ederer E 915786… "[An… NA 0
#> 5 K<c3><a4>ssler P and Breme K 915784… "[Ev… NA 0
#> 6 Brubaker Ruth B and Bay Curt and Chacon Daniel W and Carson Madelein… 911523… "93 … "Introd… 0
#> 7 Kumar Suresh and Vellymalay N 915785… "A C… NA 0
#> 8 Lock S 915784… "A D… NA 0
#> 9 Bosco Nicolina and Giaccherini Susanna and Meringolo Patrizia 911019… "A g… "This a… 0
#>10 Abd El Salam, Amira E and AbdAllah Amany M and El Maghawry Hala A 911017… "Eff… "Backgr… 0
#># ℹ 2,755 more rows
#># ℹ Use `print(n = ...)` to see more rows
# Loading INCLUDED studies
ris_dat_incl <- read_refs("friends_incl.ris") |>
suppressWarnings() |>
as_tibble() |>
select(author, eppi_id, title, abstract) |>
mutate(
human_code = 1, #Tracking the human decision
across(c(author, title, abstract), ~ na_if(., "NA"))
)
ris_dat_incl
#> # A tibble: 97 × 5
#> author eppi_id title abstract human_code
#> <chr> <chr> <chr> <chr> <dbl>
#> 1 G<c3><b6>kkaya Fu<cc><88>sun and Gedik Z 915785… A Sc… NA 1
#> 2 ACTRN12607000254493 911528… Long… "INTERV… 1
#> 3 ACTRN12615000382572 911528… The … "INTERV… 1
#> 4 Ahlen Johan and Breitholtz Elisabeth and Barrett Paula M and Gallego… 911544… Scho… "Anxiet… 1
#> 5 Ahlen Johan and Hursti Timo and Tanner Lindsey and Tokay Zelal and G… 911019… Prev… "Our st… 1
#> 6 Green Sarah L 915785… An e… NA 1
#> 7 Anticich Sarah A. J and Barrett Paula M and Silverman Wendy and Lach… 910111… The … "This s… 1
#> 8 Barker Leslie Jayne 915785… Prev… NA 1
#> 9 Barrett PM and Moore AF and Sonderegger R 911527… The … "Young … 1
#>10 Barrett PM and Shortt AL and Wescombe K 911529… Exam… NA 1
#># ℹ 87 more rows
#># ℹ Use `print(n = ...)` to see more rowsCreate the test dataset
Now that we have loaded our reference data, we can construct the test
data by randomly sampling 150 irrelevant records (as suggested in Vembye et al., 2025) and
50 relevant records that include both a title and an abstract. For this
purpose we use sample_references(). Again, this is for
illustration purposes only, as you may not yet have access to 50
relevant records at this stage of screening. We recommend identifying at
least 10 relevant references before constructing the test data. As
further described in Vembye et al. (2025), we remove all
records without abstracts, as these can distort the accuracy of the
screening.
set.seed(09042024)
excl_sample <-
ris_dat_excl |>
filter(!is.na(abstract)) |>
sample_references(150)
incl_sample <-
ris_dat_incl |>
filter(!is.na(abstract)) |>
sample_references(50)
test_dat <-
bind_rows(excl_sample, incl_sample) |>
mutate(
studyid = 1:n()
) |>
relocate(studyid, .after = eppi_id)
test_dat
#> # A tibble: 200 × 6
#> author eppi_id studyid title abstract human_code
#> <chr> <chr> <int> <chr> <chr> <dbl>
#> 1 Moes and Frea Dou… 9433823 1 Usin… Studies… 0
#> 2 Flemke Kimberly R 9432288 2 The … The pur… 0
#> 3 Daniels M Harry a… 9431426 3 A Me… One of … 0
#> 4 Schwartzman Mered… 9434093 4 Enha… New ana… 0
#> 5 White Stuart F an… 9434418 5 Call… OBJECTI… 0
#> 6 Chao-kai X U and … 9432240 6 A no… To deal… 0
#> 7 Todd Thomas C 9434199 7 THE … This ar… 0
#> 8 Kinoshita O and K… 9431762 8 Spec… BACKGRO… 0
#> 9 Stratton Peter an… 9434563 9 Comp… This ar… 0
#>10 Stevens Sally and… 9434158 10 Inte… The num… 0
#># ℹ 190 more rows
#># ℹ Use `print(n = ...)` to see more rowsHandle Your API Key
Now, we have our test data in place and can move on to the next step of the screening: generating your API key and handling it in R.
Get your API key
Before you can use the AIscreenR functions to screen
your references, you must generate your own secret API key from OpenAI.
To do so, first ensure you have an OpenAI account (If you have not
created one yet, you can sign up here. Once you
have an account, go to https://platform.openai.com/account/api-keys
and press the + Create new secret key button (see Figure 1
below) and name your key.
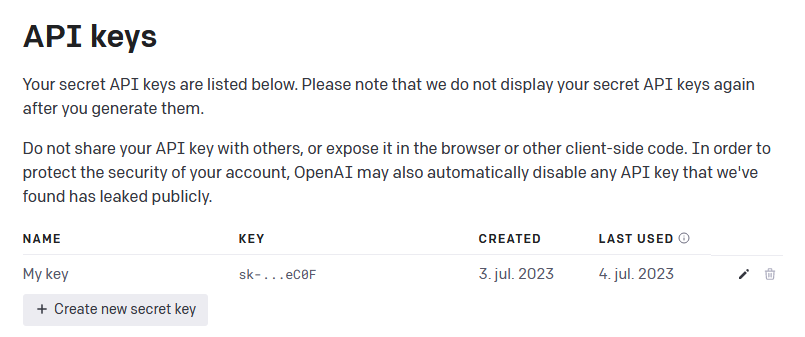
Figure 1 - Generate API key from OpenAI
Once you have generated your secret API key, remember to store it safely, as you will not be able to view it again. NOTE: If you lose your API key, you can simply generate a new one.
Manage your API key in R
NOTE: If you have already added your API key, as prescribed in the ELLMER R package, you can skip this step.
After retrieving your API key, you could theoretically add it
directly to the AIscreenR functions via the
api_key argument. However, this would be an improper way to
work the API key, as it could easily compromise your secret key. For
example, your API key would be exposed when sharing your code with
others, allowing them to access your OpenAI account. Additionally,
OpenAI will deactivate the API key if they detect that it has been
compromised (e.g., if you push it to a public GitHub repository). To
prevent this issue you have several options from here. You can either
work with what we call a permanent or a temporary solution.
Permanent solution
The easiest way to manage your API key is to add it permanently to
your R environment as an environment variable. You can do this by using
usethis::edit_r_environ().
# Run code to open your .Renviron file
usethis::edit_r_environ()In the .Renviron file, write
CHATGPT_KEY=your_key as depicted in Figure 2.
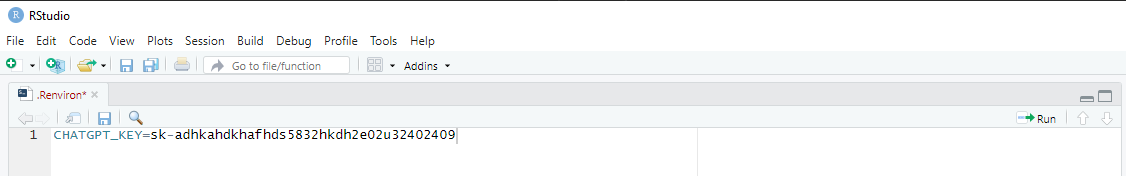
Figure 2 - R environment file
Thereafter, close and save the .Renviron file and
restart RStudio (ctrl + shift + F10). From now on, the
AIscreenR functions will automatically use
get_api_key() to retrieve your API key from your R
environment. With this approach, you will not need to worry about your
API key again—unless you update RStudio, delete the key deliberately, or
get a new computer. In such cases, you will need to repeat this
process.
Temporary solution
If you do not want to add you API key permanently to your R
environment, you can use set_api_key(). When executing
set_api_key(), a pop-up window will appear where you can
enter your API key. This will add your API key as temporary environment
variable. Consequently, after restarting RStudio, your API key will no
longer be present in your R environment. Alternatively, you can pass a
decrypted key to the set_api_key(), like
set_api_key(key = secret_decrypt(encrypt_key, "YOUR_SECRET_KEY_FOR_DECRYTING")).
For further details on this approach, refer to the HTTR2
package.
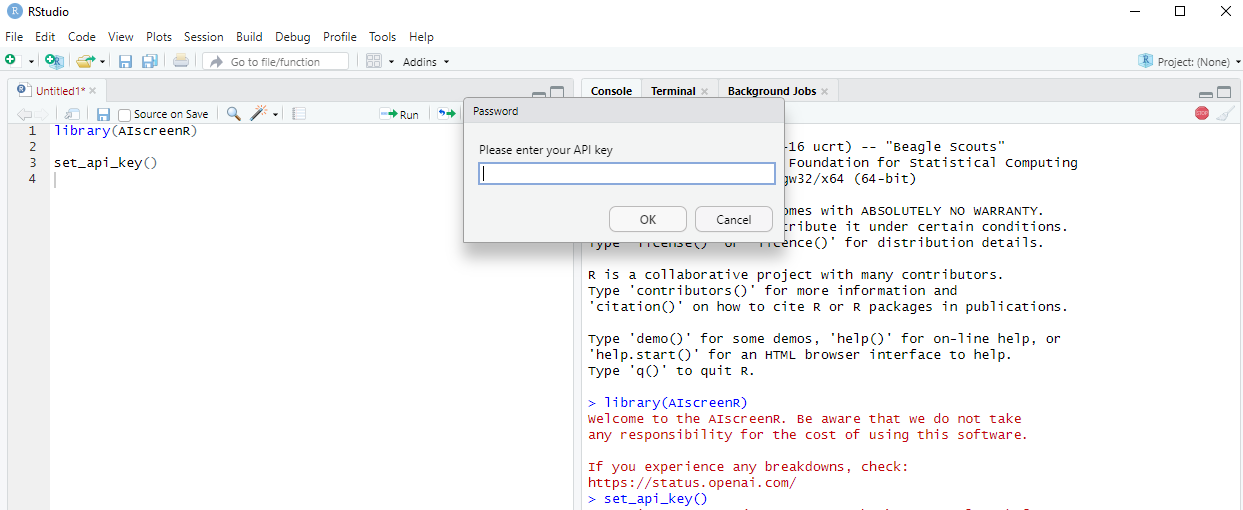
Figure 3 - Set API key
Prompting in R
The next step involves writing one or more prompts to be used for screening. This can be done in several ways. You can either write your prompt directly in R, as illustrated below:
prompt <- "We are screening studies for a systematic literature review.
The topic of the systematic review is the effect of the FRIENDS preventive programme
on reducing anxiety symptoms in children and adolescents. The FRIENDS programme is a
10-session manualised cognitive behavioural therapy (CBT) programme which can be
used as both prevention and treatment of child and youth anxiety. The study should
focus exclusively on this topic and we are exclusively searching for studies with
a treatment and a comparison group. For each study, I would like you to assess:
1) Is the study about the FRIENDS preventive programme?
2) Is the study estimating an effect between a treatment and control/comparison group?"Or you can simply write it in Word, as shown in Figures 4.
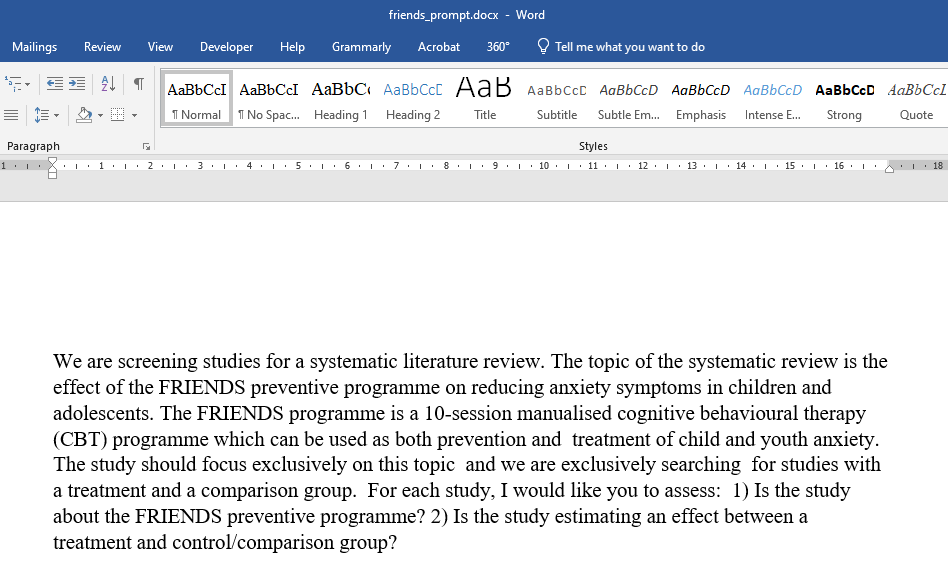
Figure 4 - Prompting in Word
Then you can load your prompt(s) via readtext() from the
readtext package.
word_path <- system.file("extdata", "word_prompt_1.docx", package = "AIscreenR")
prompt <-
readtext::readtext(word_path)$text |>
stringr::str_remove_all("\n")Run the Test Screening
Now we are actually ready to conduct the title and abstract screening
with tabscreen_gpt(). To make the
tabscreen_gpt() running, one needs to specify the following
information:
- The data with the title and abstract information.
- The prompt(s).
- The names of the variables containing the titles and abstracts.
- The model(s) you want to use for screening.
Optional
- The variable name of the variable containing the study IDs.
- The number of screenings you want to run with the given model
(Default is
1but with cheap models it can be advantageous to conduct multiple screenings and make inclusion judgments based on how many times a study has been included across the given number of screenings). - Set the request per minutes.
# Gets information about whether you have access to a given model and
# how many requests per minutes you are allow to send.
models_rpm <- rate_limits_per_minute("gpt-4o-mini")
# Set parallel plan
plan(multisession)
result_object <-
tabscreen_gpt(
data = test_dat, # RIS file data created above
prompt = prompt, # The prompt made above
studyid = studyid, # Name of variable containing study IDs
title = title, # Name of variable containing titles
abstract = abstract, # Name of variable containing abstracts
model = "gpt-4o-mini", # Model choice
reps = 10, # Using multiple screenings with the cheap gpt-4o-mini model
rpm = models_rpm$requests_per_minute # Requests per minutes retrieved from the above object
)
#> * The approximate price of the current (simple) screening will be around $0.2237.
#> Progress: ───────────────────────────────────────────────────────────────── 100%
# Back to the sequential plan
plan(sequential)We have now conducted the test screening and can get the raw results as below.
# The print output when calling the result object
result_object
#>
#> Find the final result dataset via result_object$answer_data_aggregated
result_object$answer_data_aggregated |>
select(author, human_code, final_decision_gpt, final_decision_gpt_num)
#> # A tibble: 200 × 4
#> author human_code final_decision_gpt final_decision_gpt_num
#> <chr> <dbl> <chr> <dbl>
#> 1 Lara Elvira and Mart<c3><ad>n-Mar<c3><ad>a … 0 Exclude 0
#> 2 Matsumoto Manabu 0 Exclude 0
#> 3 Jordan Ann 0 Exclude 0
#> 4 Antonova E and Hamid A and Wright B and Kum… 0 Exclude 0
#> 5 Iafusco Dario 0 Exclude 0
#> 6 Farrell L J and Barrett P M and Claassens S 0 Include 1
#> 7 Rasalingam G and Rajalingam A and Chandrada… 0 Exclude 0
#> 8 Chappel J N and DuPont R L 0 Exclude 0
#> 9 Waldrop Deborah P 0 Exclude 0
#>10 Ioana-Eva C<c4><83>dariu and Rad Dana 0 Exclude 0
#># ℹ 190 more rows
#># ℹ Use `print(n = ...)` to see more rowsScreen failed requests
It may happen that you experience that some of your screening
requests failed for some transient reasons, such as server overload or
other issues. To recover these failed requests, you can use
screen_errors(), as shown below.
result_object <-
result_object |>
screen_errors()Analyze Screening
We have now completed the test screening and want to assess whether
it is viable to use the gpt-4o-mini model as our second
screener. For the purpose, we can use
screen_analyzer().
screen_performance <-
result_object |>
screen_analyzer(human_decision = human_code) # state the name of the variable containing the human decision.
screen_performance
#> # A tibble: 1 × 9
#> promptid model reps top_p p_agreement recall specificity incl_p criteria
#> <int> <chr> <int> <dbl> <dbl> <dbl> <dbl> <dbl> <chr>
#>1 1 gpt-4o-mini 10 1 0.97 0.92 0.987 0.4 Studies have been included in at least 40% of the 10 screenings.Assess results via a benchmark scheme
But you might ask, how to assess whether the performances for of the model is acceptable as a second screener? To answer this question, we recommend assessing it through benchmarking. In Vembye et al. (2025), we developed a benchmark scheme based on typical duplicate human screening performance from 22 high-quality reviews. This scheme is presented in Figure 5.
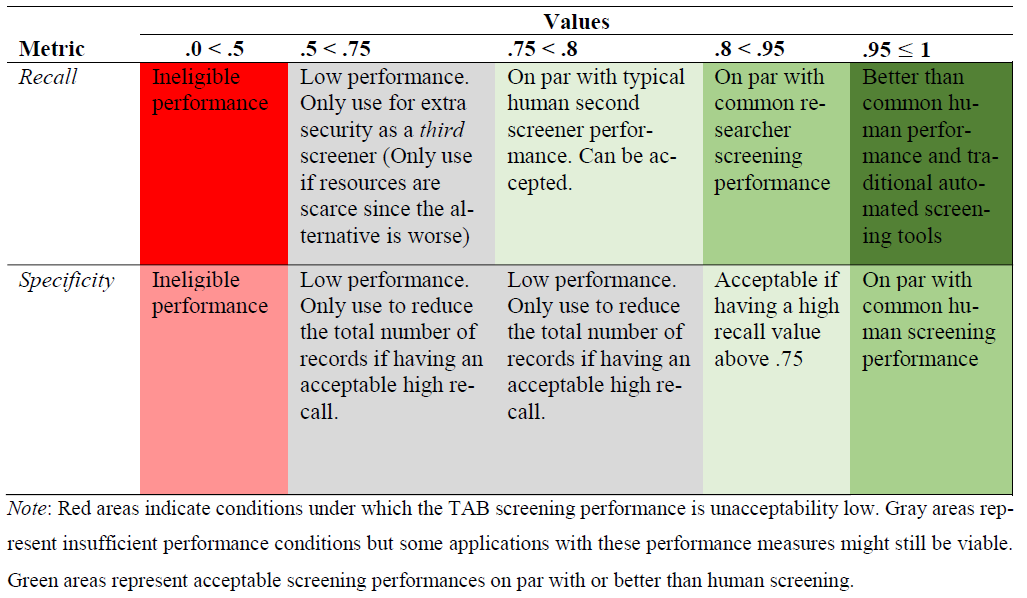
Figure 5 - Generic benchmark scheme from Vembye et al. (2025)
By using this benchmark scheme, we see that the
gpt-4o-mini model performs on least on par with typical
human screening performance. Therefore, the benchmark scheme suggests
that the model is suitable as a full second screener for the review.
Make judgments over multiple screenings
Since we have conducted multiple (i.e., 10) screenings with the
gpt-4o-mini model, we can adjust our inclusion decision
based on the number of times a study record has been included across
these 10 screenings. To obtain this information, you can use the
following code:
incl_dist <- attr(screen_performance, "p_incl_data")
incl_dist |> select(model, recall, specificity, criteria)
#> # A tibble: 10 × 4
#> model recall specificity criteria
#> <chr> <dbl> <dbl> <chr>
#> 1 gpt-4o-mini 0.96 0.987 Studies have been included in at least 10% of the 10 screenings.
#> 2 gpt-4o-mini 0.96 0.987 Studies have been included in at least 20% of the 10 screenings.
#> 3 gpt-4o-mini 0.94 0.987 Studies have been included in at least 30% of the 10 screenings.
#> 4 gpt-4o-mini 0.92 0.987 Studies have been included in at least 40% of the 10 screenings.
#> 5 gpt-4o-mini 0.92 0.987 Studies have been included in at least 50% of the 10 screenings.
#> 6 gpt-4o-mini 0.92 0.987 Studies have been included in at least 60% of the 10 screenings.
#> 7 gpt-4o-mini 0.92 0.987 Studies have been included in at least 70% of the 10 screenings.
#> 8 gpt-4o-mini 0.88 0.987 Studies have been included in at least 80% of the 10 screenings.
#> 9 gpt-4o-mini 0.84 0.987 Studies have been included in at least 90% of the 10 screenings.
#>10 gpt-4o-mini 0.74 0.987 Studies have been included in all of the 10 screenings. From these results, it appears that we can increase the model’s recall if we include a record when it has been included in at least 1 of out of the 10 screenings. In this case, we would not lose anything by doing so, since the specificity measure is rarely affected by this change.
Check and Resolve Disagreements
Even though, the gpt-4o-mini model yielded a highly
acceptable screening performance, it is useful to assess the reasons for
disagreements between the human reviewers and the GPT model. This
information can help refine your prompt(s), especially if it appears
that the GPT model is misinterpreting certain concepts.
Get detailed descriptions for disagreement records
To get detailed descriptions of GPT’s reasons for exclusion
(alternatively inclusion), you can set
decision_description = TRUE and run the screening again, as
shown below.
disagree_dat <-
result_object$answer_data_aggregated |>
filter(human_code == 1, final_decision_gpt_num == 0, incl_p == 0)
plan(multisession)
result_object_detail <-
tabscreen_gpt(
data = disagree_dat,
prompt = prompt,
studyid = studyid,
title = title,
abstract = abstract,
model = "gpt-4o-mini",
rpm = models_rpm$requests_per_minute,
decision_description = TRUE # Get detailed screening decisions
)
#> * The approximate price of the current (simple) screening will be around $0.0002.
#> * Be aware that getting descriptive, detailed responses will substantially
#> increase the prize of the screening relative to the noted approximate prize.
#> Progress: ───────────────────────────────────────────────────────────────── 100%
plan(sequential)For now, we recommend obtaining detailed descriptions only for
records where there is disagreement between the human reviewers and GPT,
as this can significantly increase the screening cost. However, with the
gpt-4o-mini model, the additional cost may be less
substantial.
Example of detailed answer
Here is an example of an abstract that was included by the human reviewers and excluded by GPT.
# Example of abstract included by the human and excluded by gpt-4o-mini
result_object_detail$answer_data$abstract[1]
#> [1] "Introduction: Many universal school-based preventative intervention trials
#> for anxiety have been conducted in Western countries. This pilot study examined
#> the efficacy and acceptability of a school-based, universal preventative program
#> for anxiety among children aged 8<e2><80><93>9 years in Japan. The program was
#> based on cognitive-behavioral therapy (CBT) and was informed by similar universal
#> programs (i.e., the Fun FRIENDS program; Barrett, 2007a, 2007b). Methods: Seventy-four
#> children from a single school were allocated to an intervention or control group.
#> The intervention comprised 10 CBT sessions, and assessments were conducted before
#> and after the program. The primary outcome measure was the Spence Children's Anxiety Scale (SCAS)
#> as children's self-report. Secondary outcome measures were the Depression Self-Rating
#> Scale for Children (DSRS-C), Children's Hope Scale (Hope),
#> Spence Children's Anxiety Scale-Parent Version (SCAS-P), and Strengths
#> and Difficulties Questionnaire-Parent Version (SDQ-P). Results: The SCAS as the
#> primary outcome showed no significant differences between the two groups.
#> In addition, DSRS-C, Hope and SDQ-P also showed no significant differences.
#> SCAS-P in the intervention group showed significant decrease compared to those
#> in the control group. Conclusion: The results of this trial study suggest that
#> a school-based universal preventative program for anxiety may have no significant
#> effects on 8<e2><80><93>9-year-old children.
#> (PsycInfo Database Record (c) 2022 APA, all rights reserved)"and this is GPT’s detailed response.
# Example of explanation for exclusion
# Seems reasonable why the records was thrown out by gpt.
result_object_detail$answer_data$detailed_description[1]
#> [1] "The study does not focus on the FRIENDS preventive programme; instead,
#> it evaluates a different school-based CBT intervention and references
#> the Fun FRIENDS program without investigating its effects directly.
#> Additionally, it assesses a general anxiety intervention rather than
#> specifically measuring the FRIENDS programme's effectiveness."As can be seen from this answer, it is reasonable why the title and abstract has not been included by GPT since it is stated in the abstract that the intervention was only informed by the Fun Friends program but is distinct from it. Although, this is not a clear-cut example, it highlights the importance of ensuring that a low recall measure is not driven by insufficient human screening.
NOTE: When re-screening disagreements, it may happen (due to uncertainty in the models) that some near-included studies will be included, even though they were excluded in the initial screening. These studies could just be added to the pool of included studies.
Approximate Price of Full-Scale Screening
When you have decided to use a GPT model as your second screener, you
will likely want to know the cost of the full screening. You can obtain
this information by using approximate_price_gpt().
# All RIS file data
all_dat <-
bind_rows(ris_dat_excl, ris_dat_incl) |> # Use RIS file data here
filter(!is.na(abstract)) |> # Only screen studies with an abstract
mutate(studyid = 1:n())
app_obj <-
approximate_price_gpt(
data = all_dat,
prompt = prompt,
studyid = studyid,
title = title,
abstract = abstract,
model = c("gpt-4o-mini", "gpt-4"), # To compare model prizes
reps = c(10, 1)
)
app_obj
#> The approximate price of the (simple) screening will be around $64.1443.
app_obj$price_dollar
#> [1] 64.1443
app_obj$price_data
#> # A tibble: 2 × 6
#> prompt model iterations input_price_dollar output_price_dollar total_price_dollar
#> <chr> <chr> <dbl> <dbl> <dbl> <dbl>
#> 1 Prompt 1 gpt-4o-mini 10 2.99 0.111 3.11
#> 2 Prompt 1 gpt-4 1 59.9 1.11 61.0 When examining the price, it becomes clear why prioritizing the use
of gpt-4o-mini is beneficial. You could conduct 100
screenings with the gpt-4o-mini model, and it would still
cost only half as much as using gpt-4.
Conduct the Full Screening
You are now ready to run the full screening! In this example, you can
do so by substituting test_dat with all_dat in
the code used to screen the test data, as shown below
# Set parallel plan
plan(multisession)
# NOT RUN
result_object <-
tabscreen_gpt(
data = all_dat, # RIS file data created above
prompt = prompt, # The prompt made above
studyid = studyid, # Name of variable containing study IDs
title = title, # Name of variable containing titles
abstract = abstract, # Name of variable containing abstracts
model = "gpt-4o-mini", # Model choice
reps = 10, # Using multiple screenings with the cheap gpt-4o-mini model
rpm = models_rpm$requests_per_minute # Requests per minutes retrieved from the above object
)
#> * The approximate price of the current (simple) screening will be around $0.2237.
#> Progress: ───────────────────────────────────────────────────────────────── 100%
# Back to the sequential plan
plan(sequential)To steer the screening process when dealing with a large collection
of RIS files, it can be beneficial to split the full dataset into
smaller batches. For example one could screen batches of 1000-2000
references at a time. You can use base::split() for this
purpose.
Convert Relevant Records Back to a RIS File
Once the full screening has been conducted, you may want to load the
relevant studies back into your systematic review software tool. To do
this, you can use write_refs() from the
synthesisr package. This function converts the data frame
containing the included references into a RIS file, which can then be
imported into the systematic review software.
incl_refs <- result_object$answer_data_aggregated |>
filter(incl_p >= 0.1)
write_refs(as.data.frame(incl_refs), file = "file_name.ris", format = "ris")If you like to convert the data to a bib file, then use
format = "bib".
Future Package Updates
In this vignette, we have demonstrated how to conduct a title and abstract screening with a GPT API model. In future versions of the package, we plan to add additional articles that will cover:
- How to run the full screening of all titles and abstracts most efficiently.
- How to fine-tune a model.
- How to use multiple-prompt screening, i.e., making one prompt per inclusion criteria instead of adding all to the same prompt.
- A new report functions to assess and resolve disagreements between human and GPT decisions.